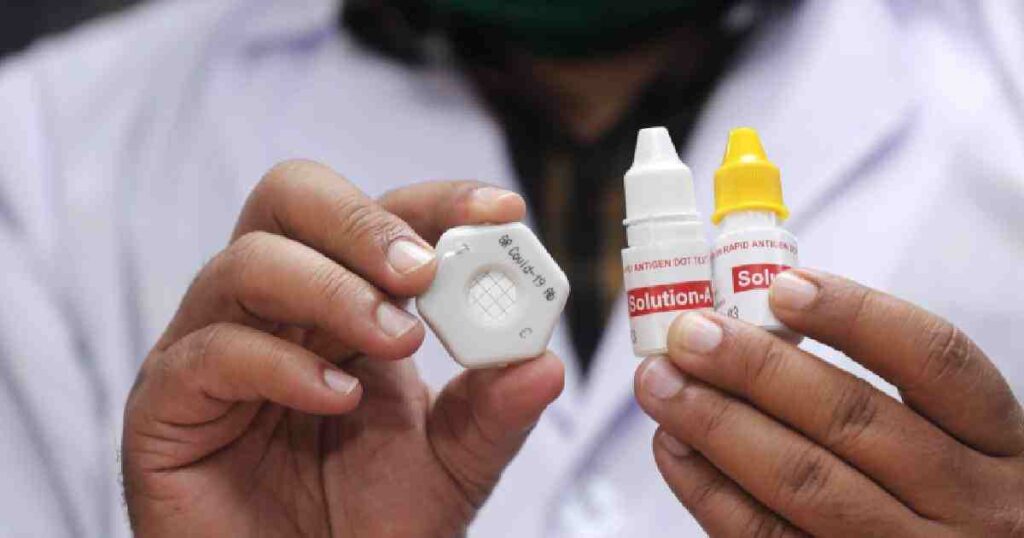
Dhaka, Sept 22 – Gonoshasthya Kendra (GK) founder Dr Zafrullah Chowdhury on Tuesday alleged that the government did not approve the coronavirus antibody and antigen kits developed by their organisation due to a ‘business motive’.“It’s a government of businessmen. So, even though our antibody and antigen kits are ready, the government has not approved those. Now they’ve allowed the import of those with a business purpose,” he said.
Zafrullah, also a freedom fighter, made the remarks while speaking at a programme at Gonoshasthya Kendra Nagar Hospital in the city’s Dhanmondi area.
He said GK will no longer apply to the government seeking approval of its kits. “But if the government approves our kits on its own, we’ll provide it with our kits.”
Zafrullah said the government is still pursuing a ‘wrong’ policy to contain the coronavirus pandemic. “They didn’t approve the corona vaccine trial in time. Gonoshasthya Kendra suffered a loss of Tk 10 crore by developing the corona kits as the government didn’t approve those. They’ve now approved the import of the kits from abroad. Such a decision has been made as the current regime is pro-businessmen one.”
Criticising the government for what he said was its failure to ‘restore discipline’ in the health sector, the freedom fighter said the country’s health system will improve only if a democratic government is established through a mid-term impartial election.”
He said the government should provide all-out support to health workers, including doctors and nurses, while they are tackling a health disaster risking their lives as frontline fighters. “Those who (doctors and nurses) sacrificed their lives in dealing with corona patients should be given the honour as national heroes and the nation must remember them.”
At the programme, the health workers of Gonoshasthya Kendra showed honour to the corona frontline fighters by clapping for a minute. A similar programme was held at the 30 centres of the GK across the country.
On May 13, GK submitted the samples of GR Covid-19 Rapid Dot Blot antigen and antibody kits to the Bangabandhu Sheikh Mujib Medical University (BSMMU) authorities for the performance trial.
The BSMMU conducted the trial of the antibody kit and found it not fully effective to detect the corona patients.
Following the BSMMU’s report, the Directorate General of Drug Administration (DGDA) did not approve the kit. – UNB